Introduction
In various sectors, it is necessary to provide certificates of conformity for produced items. These certificates attest that quality controls have been carried out to ensure that the sold item complies with current regulations.
Generate an analysis report in Odoo
With Smart Biotech, as mentioned in other articles, it is possible to configure quality controls of various types, including a 'measurement' type. For more information, I invite you to read the following article : Advanced Quality Management in Smart Biotech - Part I.
From these quality controls, it is possible to generate an analysis report. To do this, go to the list view of your quality controls and select all the controls for which you wish to generate an analysis.
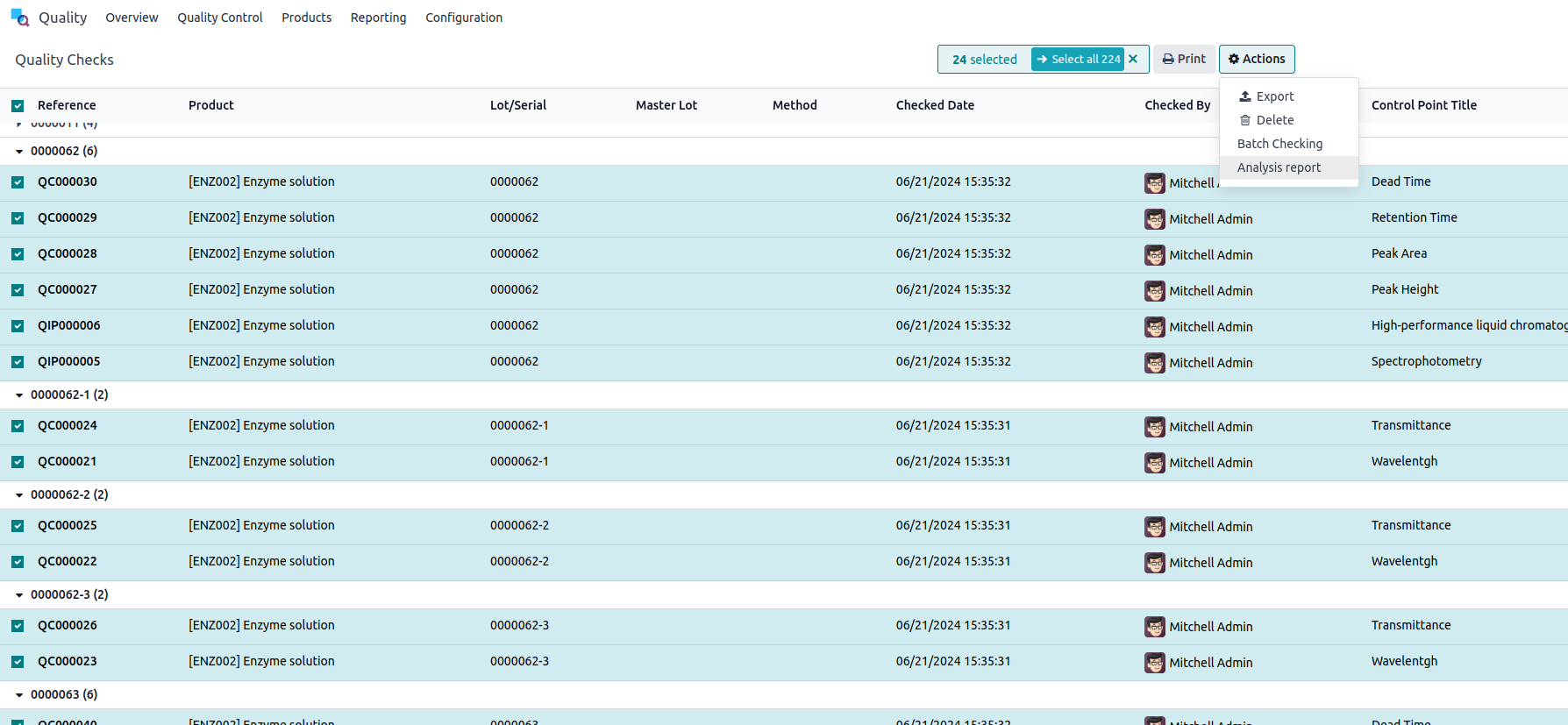
Click on Actions > Analysis Report
Upon taking this action, an analysis report will be generated.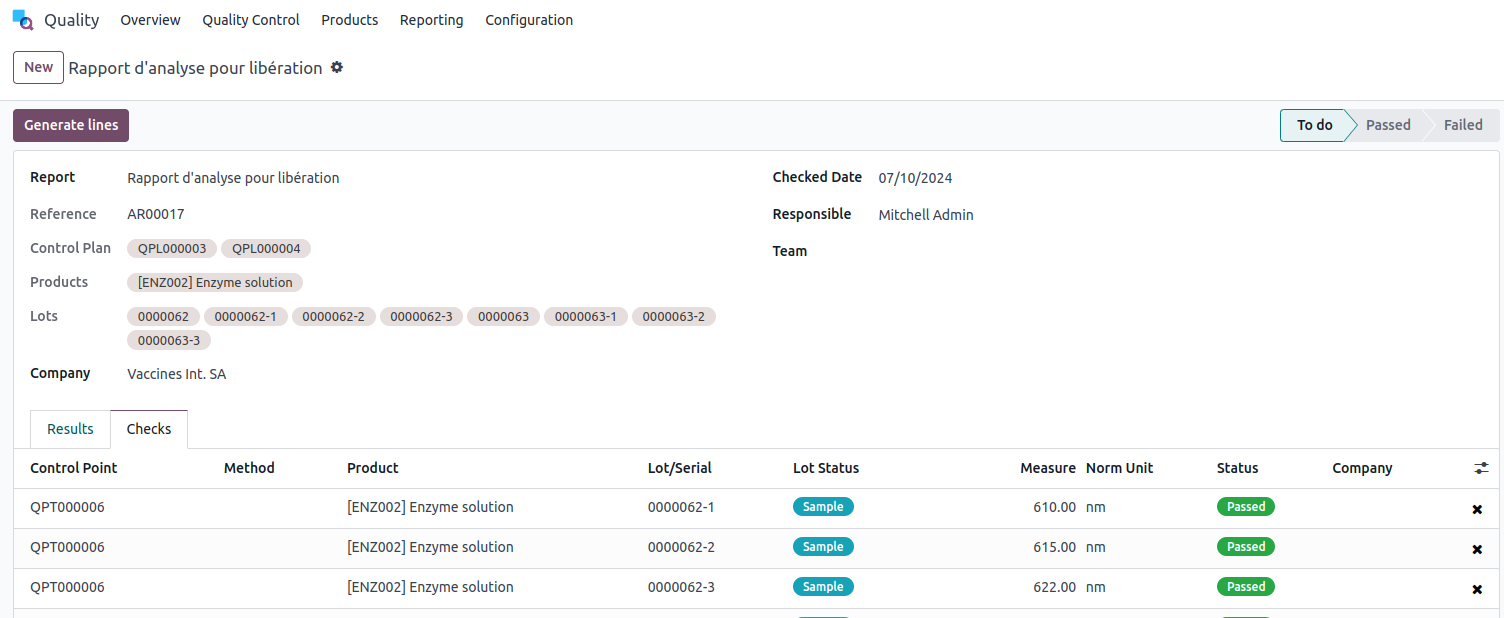
This report lists the items along with the analyzed batches. For each report, you have the option to assign a responsible person and an editing date.
Each analysis report is assigned a unique number, such as AR00017. You can control the sequence that generates the reference.
In the 'Results' tab, Odoo generates an analysis of the obtained measurements.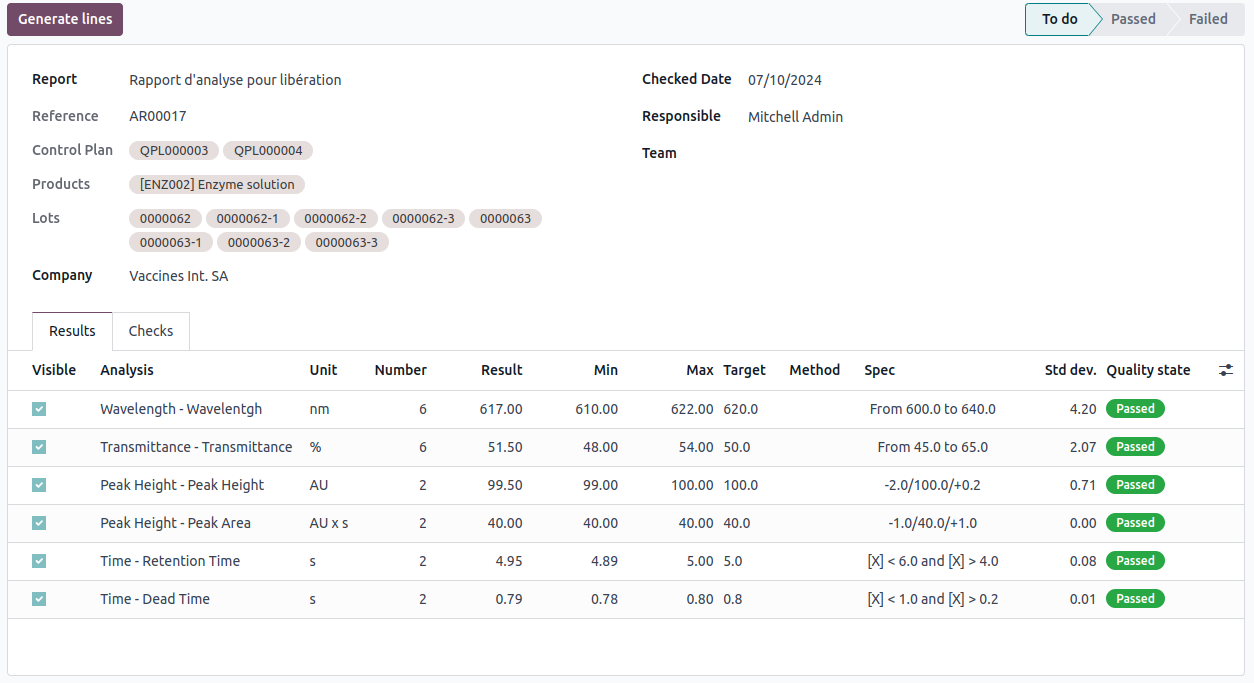
For example, the first line indicates that we performed 6 measurements of type "Wavelength". These measurements were recorded in the unit 'nm'. The expected value is 620.0 nm (Target); we obtained an average of 617 nm with a minimum of 610 and a maximum of 622. The standard deviation is 4.2. We also specify the acceptance criterion, which considers any measurement between 600 and 640 nm as passing.
If you look in the top right corner of the image above, you'll notice that the analysis report is marked as 'To do'. Specific access rights are required to approve or reject an analysis report.
Generate a certificate of conformity
Based on an analysis report, it is possible to generate a certificate of conformity.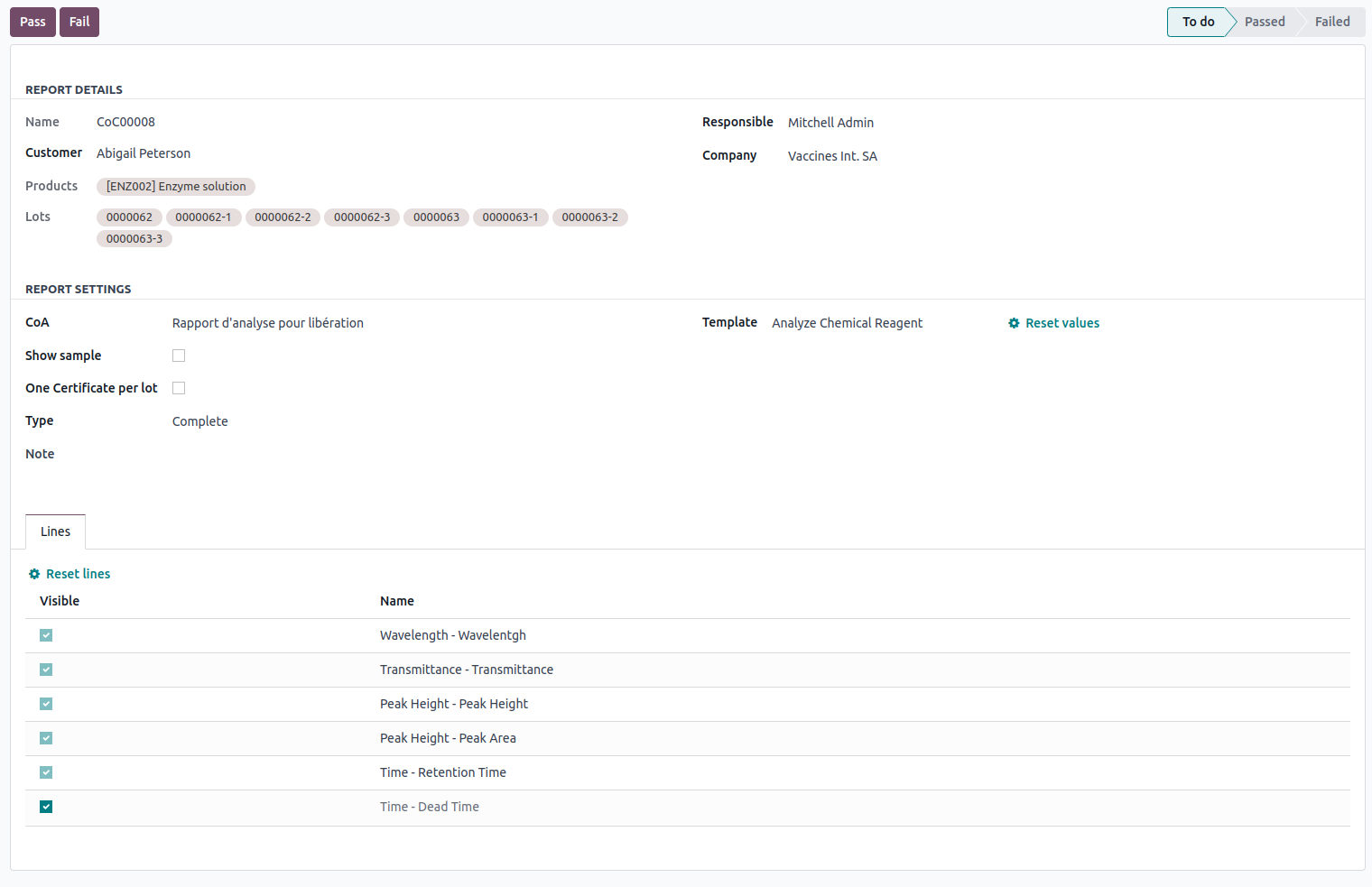
You can link multiple certificates of conformity to the same analysis report. Each certificate can be associated with a specific client. Similar to the analysis report, you can specify a responsible person and an editing date for each certificate of conformity.
You have several configuration options for the certificates of conformity (CdC).
Show sample
If checked, sample identifiers will be included on the certificate if analyses have been conducted on samples.
One Certificate per lot
If enabled, the system generates one certificate per batch. Otherwise, all certified batches will be listed on the same page.
Type
There are three types:
- Complete: The certificate of conformity includes an appendix page with the analysis of results (average, standard deviation, etc.), as well as a list of all quality controls performed with their execution dates.
- Aggregate: The certificate of conformity includes an appendix page with the analysis of results (average, standard deviation, etc.).
- Certificate of Conformity: The CoC does not contain an appendix.
Modify the certificate using Studio.
Here is what a certificate of conformity looks like.

The signature is that assigned to the responsible user on the report. You can modify the report yourself using Studio.
Signing a document via Sign
The Sign module allows you to sign PDF documents. Documents signed using the Signature .
Cependant, cette application est conçue pour signer des documents statiques qui ne changent pas dans le temps, tels que :
- Des conditions de vente
- Un contrat cadre
Dans un avenir proche, nous souhaitons permettre via Smart Biotech de signer rapidement n'importe quel document PDF stocké dans Odoo. Pour appliquer sa signature, il faudra ressaisir son identifiant et mot de passe afin d'assurer l'identité de la personne qui appose sa signature.
Nous souhaitons également imposer la signature pour certaines actions dans le système, notamment pour la libération des lots.
En savoir plus
Pour en apprendre plus sur Smart Biotech, n'hésitez pas à vous inscrire à l'une de nos séances de découverte gratuites.
To find out more about the solution, visit our product page.
Vous pouvez également nous contacter via le formulaire en ligne.
Certificate of Conformity