In today’s world, where traceability and rigorous quality testing are prerequisites for performance, compliance, and long-term sustainability, quality is no longer a question—it is a necessity. Smart Biotech, an Odoo-based solution, has been designed to meet the demanding requirements of biotech and medtech companies. Yet, the breadth of its functionalities makes it indispensable for any organization where quality and traceability form the foundation of value—from industrial processes to the food, cosmetics, and chemical sectors.
Quality: A cross-industry imperative for high-standards businesses
Quality management extends far beyond regulatory compliance. It defines reputation, customer trust, and competitiveness. In biotech and medtech, quality is first and foremost a matter of survival: complying with GxP standards, ensuring sterility, tracking every batch of raw material or finished product—all are essential to market access and risk reduction. But these needs are equally critical in other industries: In food processing, where food safety requires the traceability of every ingredient. In cosmetics, where product testing and supplier validation are decisive. In chemicals, where even minor incidents can jeopardize public health or damage a carefully built brand reputation.
Smart Biotech: A comprehensive answer to quality and traceability management
Key innovations include:
- Customizable quality statuses by batch: Each batch or series is assigned and retains a status based on its progress and quality control results. Non-compliant batches cannot enter stock zones or move forward without explicit validation.
- Control plans and groups: Define and orchestrate test protocols with multiple validation levels (per product, per operation, per quantity, or per master lot), including dual validation in regulated contexts. This ensures only complete validation can release or block a batch, eliminating ambiguity.
- Full traceability: Multi-level tracking (raw material, component, sub-batch, master lot, serial number) allows, for example, retrieval in just a few clicks of all products linked to a specific raw material batch, or detailed documentation of a component’s history following an incident.
- Advanced sampling management: Enables creation, management, and retrieval of samples directly tied to their original batch, supporting precise and fully documented test campaigns.
- Automated certificates of conformance & analysis reports: Customers automatically receive digital certificates attesting to batch or shipment compliance, with detailed test results and control dates—without additional administrative workload.
- Dynamic control frequency management: Control rules automatically adapt to past results (increasing frequency after deviations, decreasing it after consistent success), optimizing resources without compromising quality.

Example: Sample configuration in Smart Biotech.
Multi-sector applications
Although rooted in biotechnology, Smart Biotech has proven its value across multiple industries:
- Food Industry: Detailed tracking of ingredient batches, microbiological control, shelf-life management, and automated compliance certificates for retail distribution.
- Cosmetics: Supplier validation for active ingredients, traceability of clinical trials, formula substitution management, and audit-ready documentation.
- Chemicals: Batch testing orchestration, non-conformity tracking, and multi-site management with standardized practices.
- Medical Devices: GxP-compliant documentation, cross-validation at every production stage, and automatic management of blocked or sampled batches according to risk level.
A driver of compliance, continuous improvement, and competitiveness
Adopting Smart Biotech means safeguarding against regulatory deviations with robust workflows, mandatory validations, and automatic data retrieval during audits. It also saves valuable time: every operation is traced once and for all, every stakeholder moves through the workflow with confidence, and the detailed history becomes a tool for continuous improvement rather than a constraint.
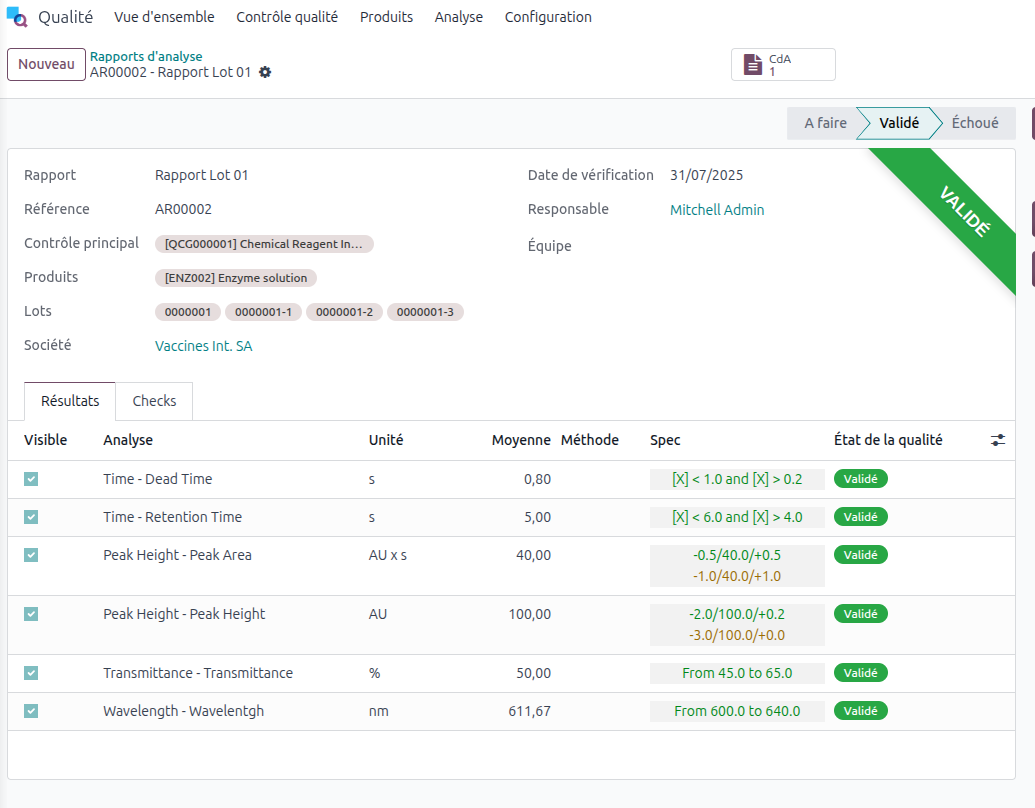 Example: Analysis report that can be issued as a Certificate of Conformance in Smart Biotech.
Example: Analysis report that can be issued as a Certificate of Conformance in Smart Biotech.
Conclusion
Quality and traceability are no longer reserved for pharmaceutical or biotech companies. In a landscape of increasingly demanding customers, stricter regulations, and reputations at risk with a single incident, Smart Biotech delivers a rigorous, expert, and universal solution. Investing in quality today means securing the future of your business—whatever your industry.
The importance of quality in Smart Biotech